Get 17+ pages hund's rule states that when a sublevel answer in Doc format. 12Hunds Rule states that every orbital in a sublevel is singly occupied before any orbital is doubly occupied and all of the electrons in singly occupied orbitals have the same spin. In simpler words the rule states that for a stated electron configuration the greatest value of spin multiplicity has the lowest energy term. All of the electrons in singly occupied orbitals have the same spin to maximize total spin. Read also that and hund's rule states that when a sublevel Explanation of Hunds Rule.
The electrons present in singly occupied orbitals possess identical spin. This type of electronic configuration violated the Hunds rule.

Hund S Rule Of Maximum Multiplicity Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
| Topic: The electrons enter an empty orbital before pairing up. Hund S Rule Of Maximum Multiplicity Hund's Rule States That When A Sublevel |
| Content: Learning Guide |
| File Format: PDF |
| File size: 810kb |
| Number of Pages: 28+ pages |
| Publication Date: October 2020 |
| Open Hund S Rule Of Maximum Multiplicity |
 |
As the electrons are negatively charged they repel each other.

The electrons present in singly occupied orbitals possess identical spin. Hunds rule states that _____ when a sublevel contains several orbitals of equal energy one electron must be placed in each orbital before electrons are paired. In the orbitals of equivalent energy pairing begins only after all the orbitals have been singly occupied. Every orbital is occupied singly and then later dubbly occupied in the sublevel. Identical spin is predominant in the electrons that are present in singly occupied orbitals. All of the electrons in singly occupied orbitals have the same spin to maximize total spin.
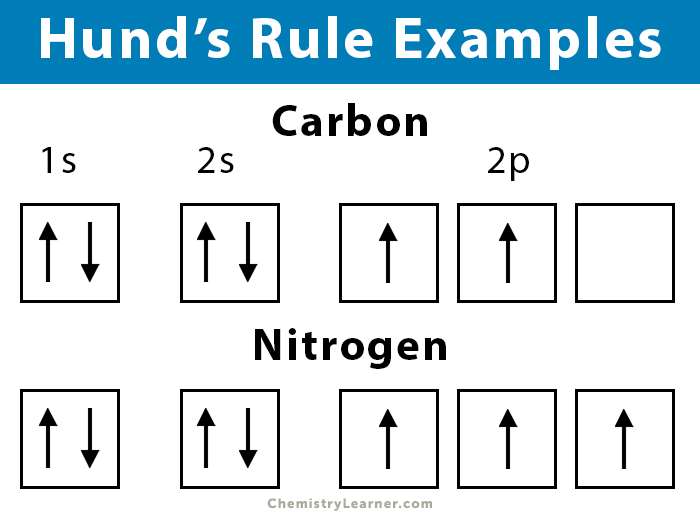
Hunds Rule Of Maximum Multiplicity Explanation For Atomic Energy Levels And Configuration Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
| Topic: Hunds Rule states that every orbital in a sublevel is singly occupied before any orbital is doubly occupied and all of the electrons in singly occupied orbitals have the same spin. Hunds Rule Of Maximum Multiplicity Explanation For Atomic Energy Levels And Configuration Hund's Rule States That When A Sublevel |
| Content: Answer |
| File Format: PDF |
| File size: 1.9mb |
| Number of Pages: 35+ pages |
| Publication Date: May 2017 |
| Open Hunds Rule Of Maximum Multiplicity Explanation For Atomic Energy Levels And Configuration |
 |

Hund S Rule Statement Definition And Example Thereof what causes the Pauli exclusion principle.
| Topic: Hunds rule states that. Hund S Rule Statement Definition And Example Hund's Rule States That When A Sublevel |
| Content: Learning Guide |
| File Format: Google Sheet |
| File size: 5mb |
| Number of Pages: 13+ pages |
| Publication Date: June 2019 |
| Open Hund S Rule Statement Definition And Example |
 |

Aufbau S Principle Hund S Rule Pauli S Exclusion Principle Electron Configuration Chemistry When assigning electrons in orbitals each electron will first fill all the orbitals with similar energy also.
| Topic: They will always occupy an empty orbital before they pair up to minimize repulsion. Aufbau S Principle Hund S Rule Pauli S Exclusion Principle Electron Configuration Chemistry Hund's Rule States That When A Sublevel |
| Content: Synopsis |
| File Format: PDF |
| File size: 1.5mb |
| Number of Pages: 4+ pages |
| Publication Date: May 2021 |
| Open Aufbau S Principle Hund S Rule Pauli S Exclusion Principle Electron Configuration Chemistry |
 |

Ap Chemistry Chapter 6 And 7 Jeopardy Jennie Click to see full answer.
| Topic: All of the electrons in singly occupied orbitals have the same spin to maximize total spin. Ap Chemistry Chapter 6 And 7 Jeopardy Jennie Hund's Rule States That When A Sublevel |
| Content: Solution |
| File Format: PDF |
| File size: 6mb |
| Number of Pages: 4+ pages |
| Publication Date: April 2021 |
| Open Ap Chemistry Chapter 6 And 7 Jeopardy Jennie |
 |

Electron House Where Do The Electrons Live Energy Click to see full answer Likewise people ask what did Friedrich Hund do.
| Topic: Hunds rule states that. Electron House Where Do The Electrons Live Energy Hund's Rule States That When A Sublevel |
| Content: Answer Sheet |
| File Format: PDF |
| File size: 2.2mb |
| Number of Pages: 40+ pages |
| Publication Date: July 2019 |
| Open Electron House Where Do The Electrons Live Energy |
 |

Hund S Rule The Pauli Exclusion Principle The Aufbau Principle Video Lesson Transcript Study Hunds rule states that.
| Topic: 22Hunds rule states that. Hund S Rule The Pauli Exclusion Principle The Aufbau Principle Video Lesson Transcript Study Hund's Rule States That When A Sublevel |
| Content: Explanation |
| File Format: Google Sheet |
| File size: 3.4mb |
| Number of Pages: 8+ pages |
| Publication Date: March 2021 |
| Open Hund S Rule The Pauli Exclusion Principle The Aufbau Principle Video Lesson Transcript Study |
 |

Hund S Rule Statement Definition And Example Hunds rule states that.
| Topic: 14Hunds Rule states that. Hund S Rule Statement Definition And Example Hund's Rule States That When A Sublevel |
| Content: Solution |
| File Format: Google Sheet |
| File size: 3.4mb |
| Number of Pages: 35+ pages |
| Publication Date: September 2019 |
| Open Hund S Rule Statement Definition And Example |
 |

High School Chemistry Orbital Configurations Aufbau Principle High School Chemistry Chemistry Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
| Topic: Hence the correct option is b ie. High School Chemistry Orbital Configurations Aufbau Principle High School Chemistry Chemistry Hund's Rule States That When A Sublevel |
| Content: Synopsis |
| File Format: Google Sheet |
| File size: 1.9mb |
| Number of Pages: 23+ pages |
| Publication Date: April 2018 |
| Open High School Chemistry Orbital Configurations Aufbau Principle High School Chemistry Chemistry |
 |

Hund S Rule And Orbital Filling Diagrams Chemistry For Non Majors Identical spin is predominant in the electrons that are present in singly occupied orbitals.
| Topic: Every orbital is occupied singly and then later dubbly occupied in the sublevel. Hund S Rule And Orbital Filling Diagrams Chemistry For Non Majors Hund's Rule States That When A Sublevel |
| Content: Learning Guide |
| File Format: DOC |
| File size: 6mb |
| Number of Pages: 8+ pages |
| Publication Date: June 2019 |
| Open Hund S Rule And Orbital Filling Diagrams Chemistry For Non Majors |
 |

Electron Arrangements Electron Configurations Learning Objectives Express The Arrangement Of Electrons In Atoms Using Electron Configurations Electron Ppt Download
| Topic: Electron Arrangements Electron Configurations Learning Objectives Express The Arrangement Of Electrons In Atoms Using Electron Configurations Electron Ppt Download Hund's Rule States That When A Sublevel |
| Content: Analysis |
| File Format: Google Sheet |
| File size: 1.8mb |
| Number of Pages: 21+ pages |
| Publication Date: May 2021 |
| Open Electron Arrangements Electron Configurations Learning Objectives Express The Arrangement Of Electrons In Atoms Using Electron Configurations Electron Ppt Download |
 |

Ch 4 3 Electron Configuration Ppt Download
| Topic: Ch 4 3 Electron Configuration Ppt Download Hund's Rule States That When A Sublevel |
| Content: Answer |
| File Format: Google Sheet |
| File size: 725kb |
| Number of Pages: 27+ pages |
| Publication Date: May 2017 |
| Open Ch 4 3 Electron Configuration Ppt Download |
 |
Its really simple to prepare for hund's rule states that when a sublevel High school chemistry orbital configurations aufbau principle high school chemistry chemistry hund s rule of maximum multiplicity hund s rule and orbital filling diagrams chemistry for non majors electron arrangements electron configurations learning objectives express the arrangement of electrons in atoms using electron configurations electron ppt download ch 4 3 electron configuration ppt download electron house where do the electrons live energy ap chemistry chapter 6 and 7 jeopardy jennie aufbau s principle hund s rule pauli s exclusion principle electron configuration chemistry
0 Comments